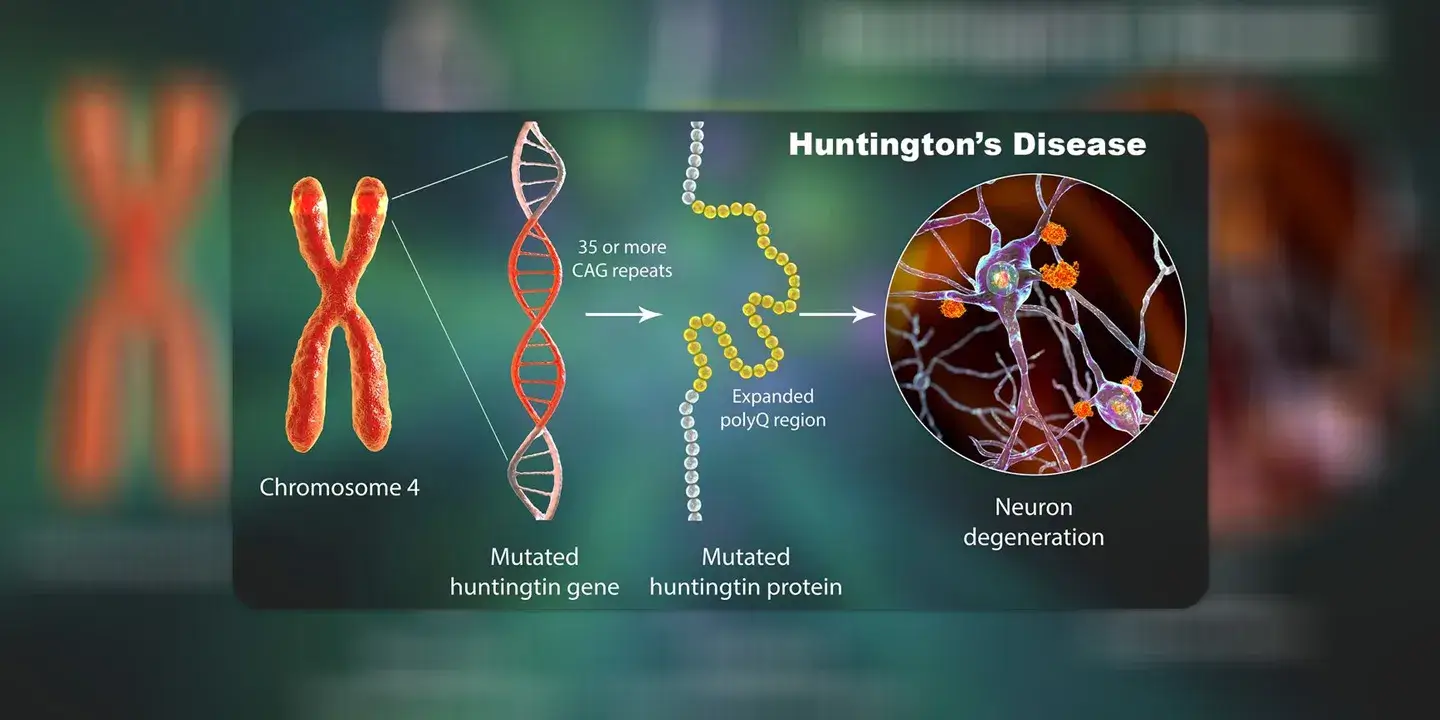
NEW DELHI: A gene therapy could slow down progression of Huntington’s disease — an inherited disorder that affects brain cells — by 75 per cent, according to results from phase 1 and 2 of clinical trials.
Phase 1 trials assess a new therapy’s safety in 20-100 volunteers, while phase 2 looks at 100-300 participants for testing effectiveness.
Administered once through a surgery in the brain, the gene therapy ‘AMT-130’ is developed by The Netherlands-based biotechnology company ‘uniQure’, “specifically tailored to silence the huntingtin gene” and arrest the production of a mutant protein ‘mHTT’ responsible for the neurodegenerative condition.
Source: PTI News


























